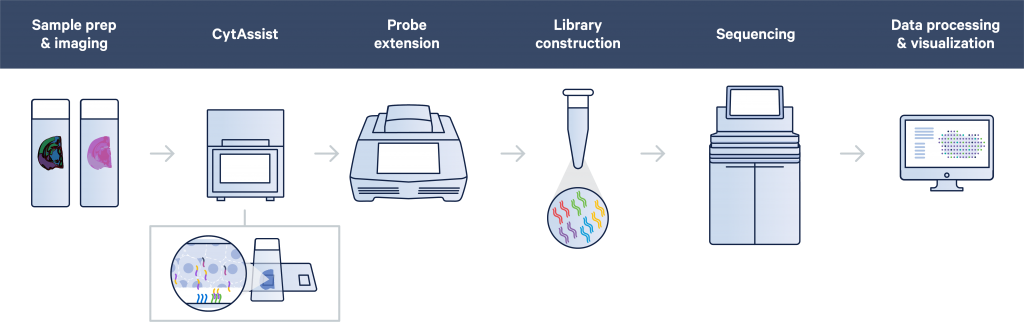
10X Genomics Visium CytAssist for FFPE samples
We now offer spatially resolved transcriptomics through the 10X Genomics Visium CytAssist, which combines histology with probe-based transcriptomics in a spatial context.
Coupling gene expression studies with tissue morphology is critical to our understanding of biology and the progression of disease. The RNA-templated ligation (RTL) probe sets from 10X Genomics allow spatial analysis of formalin-fixed paraffin-embedded (FFPE) human and mouse tissues.
We offer this protocol in combination with the CytAssist instrument, giving you the freedom to choose from two different sizes of sections, to section areas of interest within FFPE tissue samples in your own lab, place them on superfrost slides, and send those to us for processing.
Please contact us before planning your experiments for a discussion.
IMPORTANT! It is crucial that the date for sectioning is coordinated with NGI prior to sample delivery so that we can organise the first steps of library preparation within the timeframe recommended by 10X Genomics.
Sample requirements
- This method is probe-based and is only available for human and mouse tissue samples.
- We require you to check that the morphology of the sections is good enough for cell type annotations. This can be assessed by H&E staining of sections consecutive to the ones you submit to NGI. Please send us the images that you obtained highlighting your region of interest, prior to sample delivery. We will use this as a reference when selecting the areas from where the RNA should be transferred.
- Please extract RNA from the same tissue block from which sections for Visium will be taken. We require you to estimate the DV200 of the extracted RNA (on a BioAnalyzer or similar). This value should be greater than 30% to maximise chances of success at library preparation.
- Only one tissue section should be placed per slide!
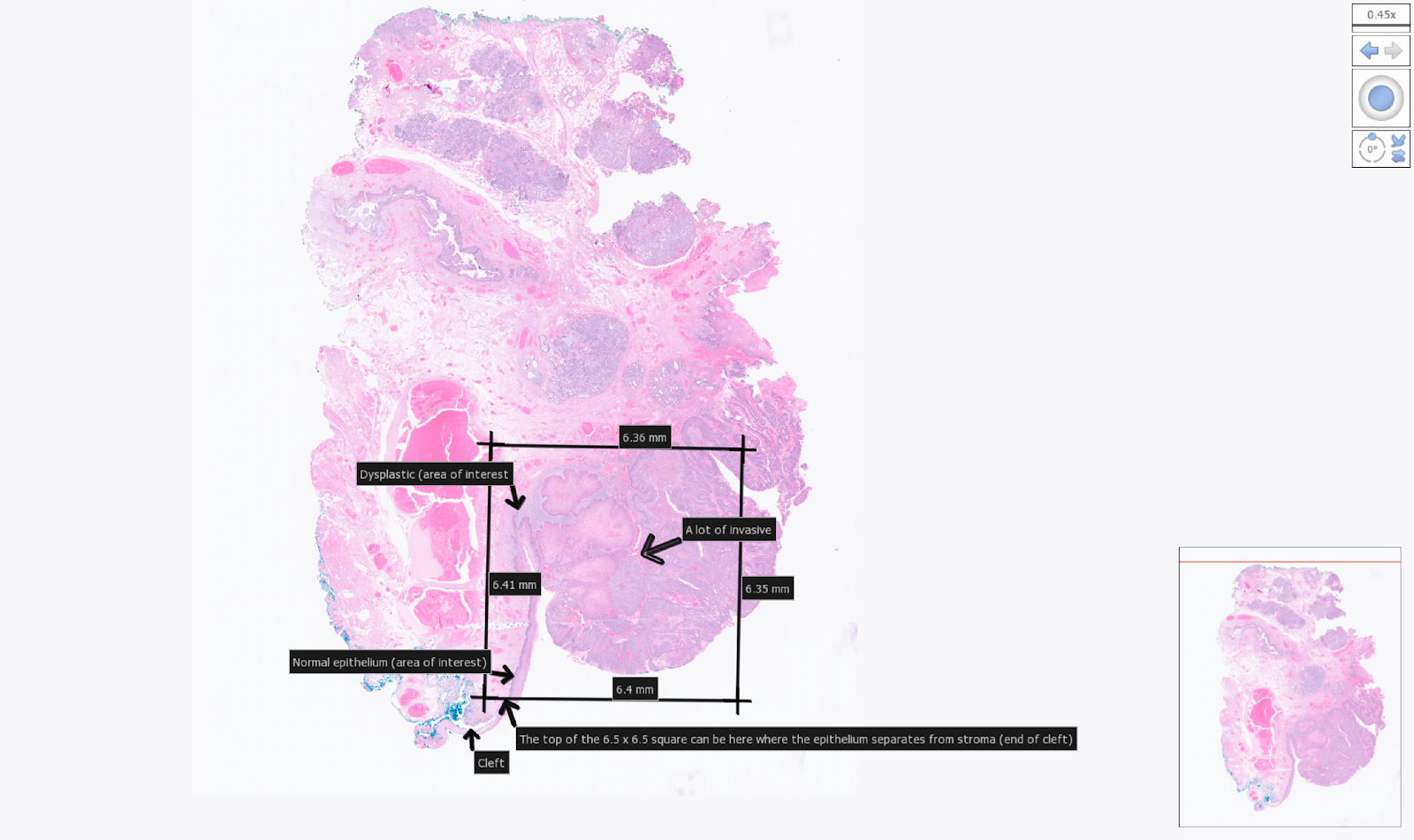
Example tissue section placement for a 6.5 mm CytAssist. Areas of interest within the section were indicated by the user with arrows.
Plan of action
- There are two types of Visium slides with different sizes of the capture areas. The user should choose the option that is most suitable for their project prior to it being set up:
- 6.5 mm x 6.5 mm (1 kit contain a total of 4 capture areas)
- 11 mm x 11 mm (1 kit contain a total of 2 capture areas)
- We require you to section and place the FFPE sections on coated or charged glass slides e.g VWR SuperFrost Plus Slides.
- Place only one section per slide.
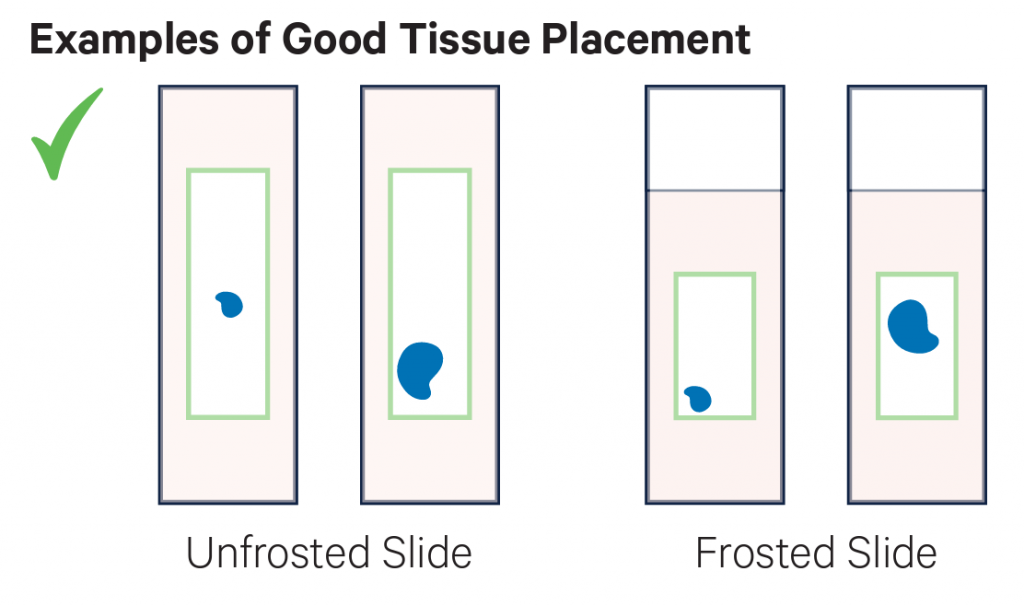
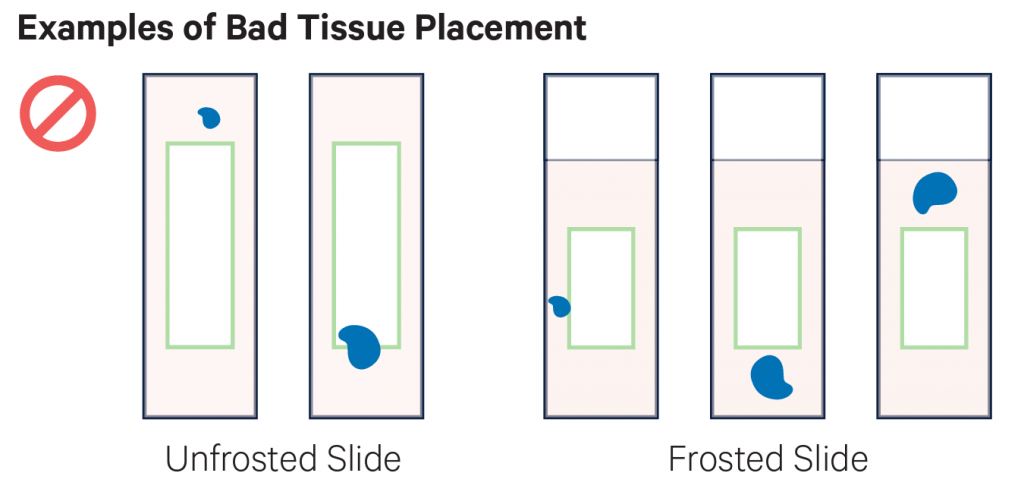
- Sections must be placed within the specific margins described here (or in the following video) sections outside the margins cannot be successfully transferred.
- To ensure that the section is in the correct area, we suggest using this printable template.
- The sections placed within the given margins may be larger than the capture areas (see sizes above). However, note that any information for tissue sections placed outside the defined areas will not be transferred onto the Visium slides during processing.
- Sections must be between 3-10 µm thick. We most often work with sections that are 5 µm thick (refer to this list of FFPE tissues tested by 10X Genomics for more information).
- After the sections are taken, the slides must be incubated at 42°C for 3 h and dried overnight at room temperature in a desiccator. Store them at room temperature in a desiccator prior to shipment to NGI.
- We recommend that you provide us with a back-up sample for each sample that is submitted to us. These will be returned to you upon request.
- Library preparation must be started within 2 weeks after sectioning and this needs to be coordinated with NGI.
- Check 10X Genomics website for further information about Visium CytAssist Spatial Gene Expression FFPE tissue preparation guide.
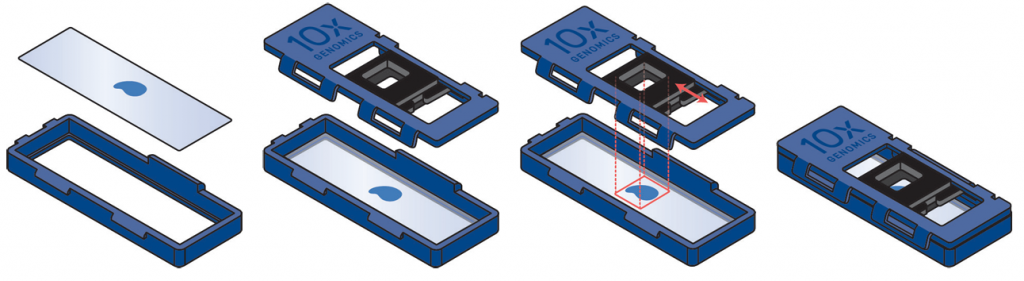
Examples of placement of sections. On the top row there examples of good placement of sections within the margins of the slides. In the middle row there are examples of tissues placed partially or totally outside the margins, and therefore they cannot be used. The bottom row shows the cassette used to transfer the probes to the Visium slide.
Information we need prior to sample delivery
- Once your order has been accepted, you will receive a project Agreement. Please read it to make sure it corresponds to your specifications and if everything looks ok please sign it and return to us electronically.
- A filled-in safety declaration that is sent to you along with the user agreement.
- You will also receive a Sample Information Sheet to fill in the sample names and the DV200 values derived from sections you analysed. Please send it back to us.
- You need to send us microscope images of H&E stained sections adjacent to the samples as previously described, clearly highlighting the area of interest (see example below).
- We will send you slide containers with the specific barcodes for the project, which should be used to send the slides to us.
Contact us prior to sectioning to coordinate timing and ensure lab availability so that library preparation can be started within the recommended 2 weeks from sectioning.
Instructions for sample labelling and delivery
Use the pre-labelled slide containers to send us the superfrost slides with your sections – we issue one container per sample that is ordered. Please place your slide with the section of interest and one backup slide in the same slide container for each sample.
The project-specific barcode label is a combination of your project ID (Pxxxxx) – see user agreement – and a serial number: e.g. `PxxxxxP1` for the first container. Each slide needs to be labelled with the NGI sample IDs from the Visium sample information sheet using xylene resistant stickers or markers, e.g.:
- Container `PxxxxxP1` will contain slides labelled `Pxxxxx_101` and a back-up slide.
- Container `PxxxxxP2` will contain slides labelled `Pxxxxx_201`and a back-up slide.
The slide containers can be shipped to us in a regular padded envelope at room temperature. However, this can strongly depend on how the slides have been previously stored, on the potential duration of transit, and on environmental conditions during shipping. For e.g. ship the samples at 4°C during a warm summer.

Example of slide container. The image shows the slide container that we will be send to the users. It has a label with the barcode corresponding to the project. Inside, they can fit a maximum of 4 slides and which should be individually labelled with the name of the sample.
Summary of Procedures at NGI
Tissue sectioning is not included in our services using the CytAssist protocol, but the following are:
- Deparaffinization, H&E staining and imaging
- Transfer of RNA-ligated probes to Visium slides using CytAssist
- Library preparation
- Sequencing
- QC (and analysis)
Sample and library preparation
The samples will be first deparaffinized, H&E stained, and imaged. This will be followed by de-crosslinking and probe hybridisation. The hybridised probes will then be transferred to the Visium slide using the CytAssist instrument. Probe extension with integration of spatial barcodes will be then performed on the slide, and the subsequent steps are carried out in reaction tubes.
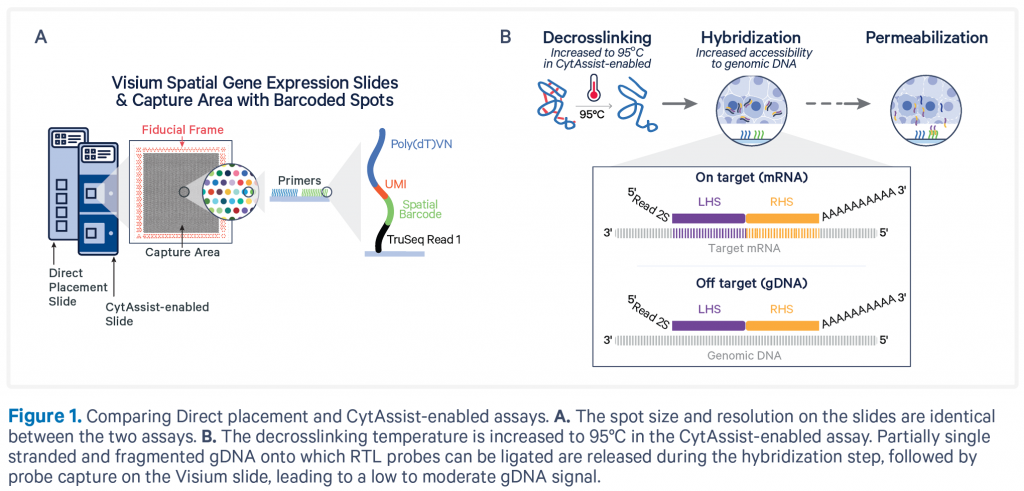
Library QC and sequencing
All individual libraries will be assessed for their quality, this includes concentration measurements and estimation of average fragment lengths. The libraries will be pooled based on these results, and later sequenced on Illumina systems such as the NextSeq or NovaSeq, depending on the sequencing depth required.
A general recommendation is a minimum of 25 000 read-pairs per spot covered by tissue (e.g. if the tissue covers the whole capture area with 5 000 spots, about 125M read-pairs per section is recommended). Thus, we may need to adjust the sequencing setup after we know the actual tissue coverage and result of library QC. Sequencing is carried out using a read setup with 28 cycles in read 1, two index reads with 10 cycles in each and then 50 cycles in read 2.
Bioinformatics
Users are strongly encouraged to submit one back-up for every sample that is submitted.
Sequencing data QC is handled by the NGI and runs through the 10x Genomics Space Ranger analysis pipeline. Raw data and Loupe visualisation files are delivered. You can read more about Spatial Transcriptomics analysis here.
Policy for damaged and/or misplaced sections
NGI will proceed with library preparation unless we deem user-supplied sections to be damaged, sub-optimally placed on slides, or not at a suitable thickness for the method. Therefore, it is in the best interest of users to ensure that our stated sample requirements are strictly adhered to.
Signing an NGI-issued agreement for your project signifies that you accept that our staff can decide how to proceed with samples that are submitted to us. Decisions may include but are not limited to:
- Proceeding with samples that do not strictly fulfill our sample requirements but have a fair chance of yielding libraries (albeit at a lower quality than would otherwise be expected), etc.
- Charging a flat fee of SEK 5000 as a processing fee for every back-up sample that is processed at NGI if the user has not adhered to our sample requirements for section placement on slides.
- Aborting projects comprising samples that are suboptimal for Visium.
Note that we can never guarantee any outcomes of library preparation or the preceding steps for the Visium protocol, especially if samples do not meet the criteria defined by 10X Genomics and that we outline.
Last Updated: 1st July 2024