10X Chromium Flex Gene Expression (fixed RNA)
Single-cell profiling of gene expression levels on fixed cells/nuclei.
In the past, most single cell assays required live cells which could make the logistics challenging. However, with the probe-based 10X Chromium Flex Gene Expression assay, users are now able to fix and store their samples before further processing, allowing increased flexibility.
IMPORTANT! Flex gene expression is only compatible with human and mouse samples.
Flex is a transcriptome wide, probe-based assay in which two adjacent probes per site are hybridised to the RNA prior to GEM-generation. In the GEMs, probes are ligated and the product is barcoded and amplified for library preparation.
Because of this, only endogenous genes which are included in the probe set can be detected (non-coding genes are not covered). A full list of probes can be found here. Information about SNVs or isoforms is lost during the assay since only ligated probes are sequenced. For the same reason, Flex can not yield information such as V(D)J or guide RNA sequences. In order to detect any exogenous genes, custom probes can be designed and included (not supported by 10X Genomics, but further information can be found here).
Call for collaboration on sample preparation of fixed tissue and FFPE material for Flex
We are planning an evaluation of cell dissociation/nuclei isolation of fixed tissue and FFPE scrolls for single cell gene expression analysis using the 10X Genomics Flex chemistry. We are looking for collaborators that are interested in analysing cells/nuclei from fixed tissue or FFPE material and could supply human or mouse material in excess for testing. We will provide:
– Free cell dissociation/nuclei isolation
– Flex analysis at subsidised cost
– Upstream data analysis.
Contact us for more information!
GEM-X Flex/ Flex Apex
With the recently released GEM-X chemistry, up to 20,000 cells can be analysed per sample, and up to 320,000 cells per reaction, dramatically decreasing the cost per cell. The GEM-X chemistry also provides lower multiplet rates, up to 80% cell recovery and fewer emulsion failures. Due to changes in kit buffers to maximise cell retention, the starting material can be as little as 25,000 cells per sample.
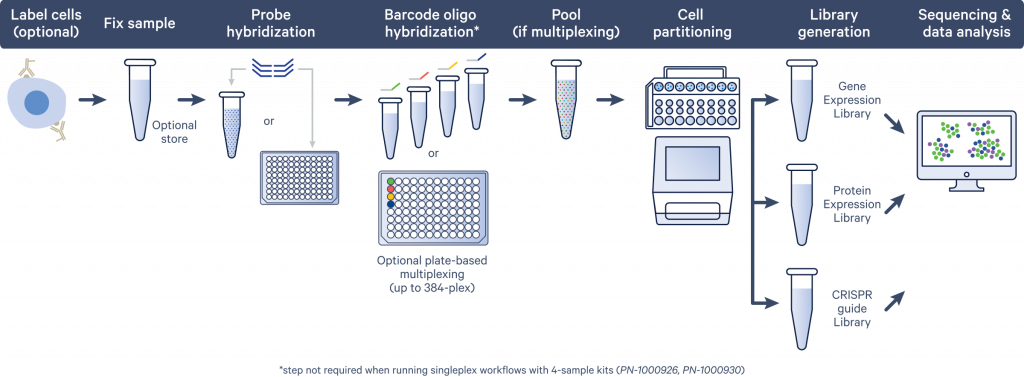
Figure adapted from https://www.10xgenomics.com/products/flex#how-it-works
Sample requirements
- Please see our full sample requirements information here.
- Start with high quality samples (>80% viability, free of debris). The 10X Flex assay is robust even for samples with lower viability (50%) but lower viability samples may have more variable cell calling and lower sensitivity. Sample quality is directly correlated to data quality.
- The maximum recommended cell size is 30 µm, larger cells may clog the microfluidic channels of the 10X Genomics chips. If cells are > 30 µm, nuclei isolation should be performed.
- We recommend starting with 1 x 10^6 cells for fixation whenever possible. For GEM-X sample fixation, the recommended minimum of fresh cells or nuclei is 25,000.
- For proceeding with probe hybridisation we require a minimum of 25,000 cells or nuclei. We recommend delivering more than this to ensure there is enough for washing and counting. If possible, we recommend delivering > 300,000 cells/nuclei.
- Samples need to be fixed following the 10X Genomics protocol for long-term storage (see protocols below).
- Make sure to use the GEM-X Flex fixation kit for maximum sample retention, as previous versions of the Flex fixation kits were not optimized for low sample input.
Compatible sample types
- Cells/Nuclei. Protocol: Fixation of Cells & Nuclei for GEM-X FLEX gene expression
- Tissue samples. Protocol: Tissue Fixation & Dissociation for GEM-X FLEX gene expression. This protocol is preferred if you do not have an optimised tissue dissociation protocol for single cell suspensions.
- FFPE blocks. Protocol: Isolation of Cells from FFPE Tissue Sections for GEM-X FLEX gene expression
- Blood. Protocol: Blood fixation and cell isolation for GEM-X FLEX gene expression
Sample Multiplexing
The Flex probes sets are available with up to 384 sample-specific barcodes, making it possible to multiplex up to 384 samples per reaction. Up to 20 000 cells/nuclei can be targeted per probe barcode. The number of different probe barcodes dictates how many cells that can be targeted per reaction in total.
10X Genomics recommends targeting 4000 cells per barcode as a starting point, with 1,6% undetectable multiplets.
Feature barcode technology for protein detection
The GEM-X Flex protocol is compatible with measuring cell surface protein or intracellular protein expression by using antibodies conjugated to a Feature barcode oligonucleotide, such as Totalseq-C antibodies (additional kits are required). Proteins need to be labelled with oligonucleotide-conjugated antibodies prior to sample fixation and delivery to NGI.
Antibodies for protein staining need to be purchased separately. Pre-conjugated antibodies are offered by for example BioLegend (Totalseq-C antibodies) and Proteintech who offer a panel of pre-conjugated antibodies targeting more than 300 proteins. For information on staining protocols, conjugation etc, see Cell Surface & Intracellular Protein Labeling for GEM-X Flex Gene Expression
Source: CG000527_ChromiumFixedRNAProfiling_MultiplexedSamples_UserGuide_Rev_E.
Starting up a single cell Flex project with NGI
- Contact us to discuss your project.
- Read our user guidelines (NGI Stockholm)
- If possible, perform a pilot of the sample preparation and fixation to ensure the required sample quality and quantity is achieved.
- Fix your samples following the 10X Genomics protocol for long-term storage. Please note that you will need to purchase all required reagents for fixation yourself, including the Chromium Single Cell Fixed RNA Sample Preparation Kit.
- Store the samples after fixation at -80ºC, in clearly labelled tubes. All samples should be shipped on dry ice.
- Submit an order via the NGI order portal, using the “Single-cell library preparation and sequencing” order form (you’ll need to make an account first, if you do not have one already). Book a day to bring your samples to us.
Information we need prior to sample delivery
- A signed agreement and filled-in sample information sheet (both provided to you when we have processed your submitted order).
- Viability counts pre-fixation and any other relevant QC information for the sample if available.
What we do with your samples
Once your samples arrive at NGI, we start by counting your cells using a fluorescent stain to make sure that the samples meet our requirements. You may choose to be present during counting.
If the samples fail this quality control step, we will only continue with the samples if we have received written confirmation, prior to sample delivery, from you that you would like to continue with the samples no matter the counting outcome. In this case, we will charge you for all of the 10X reactions that were used. If it was decided to not continue with your samples, we will not charge you anything.
If the samples pass the initial quality control, we will continue with the rest of the protocol.
Library preparation
The library preparation consists of several steps:
- Probe hybridization
Left-hand side (LHS) and right-hand side (RHS) probes are hybridized to complementary RNA molecules.
- GEM generation and barcoding
Unhybridized probes are washed away before creating an emulsion containing barcoded gel beads and cells. Cells are counted before the Gel Bead-in-Emulsion (GEM) generation is performed on the Chromium controller.
- GEM recovery and pre-amplification
The emulsion is broken by adding a recovery agent and ligated probe pairs are pre-amplified.
- Fixed RNA – Gene expression library construction
Each sample receives a sample-specific index during a final round of amplification.
Library QC and sequencing
In this step, we evaluate the yield and size distribution of the libraries and inform you about the QC status of each sample. Once the libraries have passed this QC step, they are queued for sequencing. The sequencing will be carried out following the setup stated in the agreement.
Bioinformatics
Basic bioinformatic analysis using the CellRanger software from 10X Genomics.
Last Updated: 26th February 2026