10X Genomics ATAC
Profiling of chromatin accessibility at the single cell level.
We offer the 10X Genomics Assay for Transposase-Accessible Chromatin using sequencing (ATAC-seq) which can be used to analyze genome-wide chromatin accessibility in 500-10,000 cells in a single sample. ATAC-seq can be used to study accessibility at transcription factor motif sites and thus, can give an understanding of regulation of gene expression. Using the 10X Genomics scATAC kit, the nuclei are transposed in a bulk solution and subsequently partitioned into a Gel Beads-in-emulsion (GEMs) using a microfluidic chip. Employing a pool of approximately 750,000 10x Barcodes, the transposed DNA of each nucleus is uniquely indexed. Following library generation and sequencing, the 10x Barcode is used to assign individual reads to individual nuclei.
Compatible sample types
The 10X scATAC-seq kit is only compatible with nuclei suspensions.
Sample requirements
In order to obtain high quality data from the experiment the sample should have:
- No observable debris or aggregation.
- No inhibitors of reverse transcription or GEM generation.
- A viability of the cell suspension of more than 90% (before nuclei isolation)
- Intact nuclei of >90%
- Intact nuclei with high-quality nuclear membranes and <5% of intact cells. (nuclear membranes are well resolved and nuclei show no visible sign of blebbing)
- A concentration of 155-7,700 nuclei/μl (concentration depends on targeted nuclei recovery; see table below)
We recommend performing a trial prep and assessing nuclei quality at 60x in a microscope. The quality of the nuclei suspension to be used in ATAC assay plays a crucial role in obtaining high quality sequencing data of captured nuclei. Please visit the 10X Genomics webpage for “Demonstrated Protocols” for guidance on how to obtain high quality single nuclei suspensions.
Starting an ATAC project with NGI
- Contact us at support@ngisweden.se to discuss your project.
- Read our current user guidelines here (v5).
- Submit a project request through the iLAB system (search for the ESCG facility). A project coordinator will set up your project and get back to you. Please check the project setup to make sure it reflects your specifications and if everything looks correct, agree to the terms and conditions within iLAB.
- If possible – run a pilot for the sample preparation to ensure that it yields high quality cell suspensions/nuclei.
- Book a day/days to bring your samples to us.
Information we need prior to sample delivery
- An electronically accepted project request in iLAB, with no remaining questions about setup or number of samples.
- A filled-in safety declaration that is sent to you along with the user agreement (if applicable).
Sample processing at NGI
The experiment includes the following steps:
- Nuclei suspension QC and counting
- Generation of ATAC DNA fragments including QC
- ATAC library preparation including QC
- Sequencing and data analysis
Upon delivery of the samples to NGI, the responsible lab staff will count the nuclei and check that the nuclei suspension is free of aggregates before you leave. It is your responsibility to ensure that the nuclei are of high-quality.
Should the sample be sub-optimal and you still wish to continue with the experiment, we will ask you to confirm this in writing. In such a situation you will be charged the cost of the 10x reactions regardless of the outcome. If you decide not to continue, there will be no charge unless a kit was purchased specifically for you (communicated to you before setting up your project).
All sample manipulation such as labeling, multiplexing etc must be performed by the researcher prior to delivery of the sample. The delivered sample should be ready to load on the 10X Chromium instrument.
Library preparation
1. Transposition
A transposition mix, containing a transposase, is added to the nuclei suspension. The transposase inserts its adapters preferentially into open chromatin regions which can later be amplified and sequenced.
2. GEM generation and barcoding
The samples are loaded on the Chromium X controller for GEM generation. Fragments containing a transposase-added adapter receive a 10x Barcode and are amplified inside the GEMs. The barcodes will be used to bioinformatically assign fragments to single nuclei.
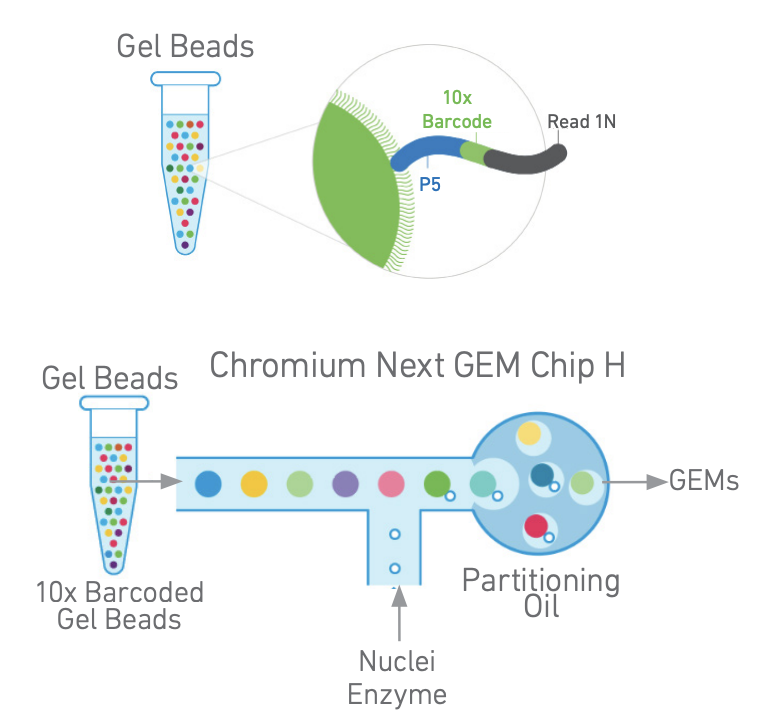
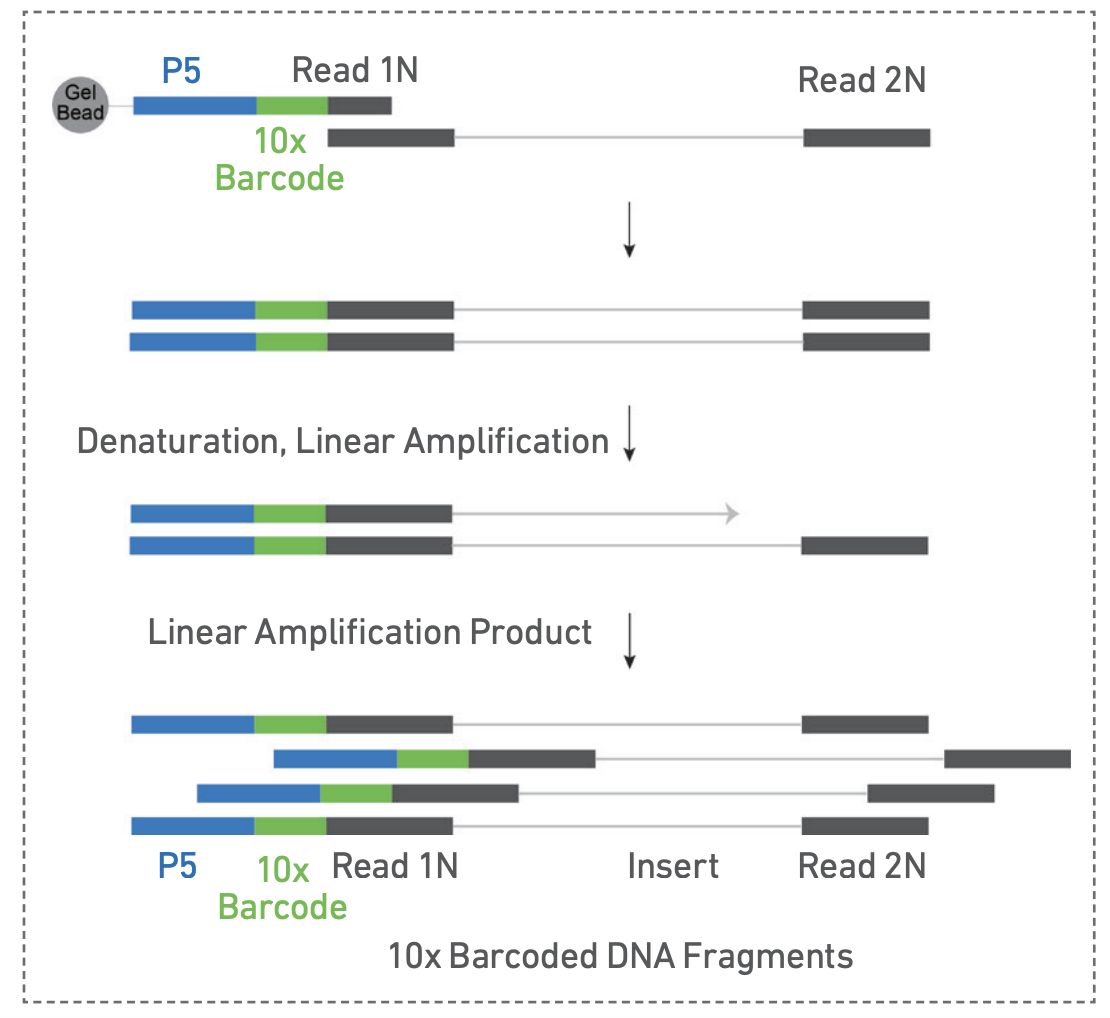
GEM generation (left). 10x barcode incorporation (right). Adapted from https://cdn.10xgenomics.com/image/upload/v1666737555/support-documents/CG000496_Chromium_NextGEM_SingleCell_ATAC_ReagentKits_v2_UserGuide_RevB.pdf
3. Post GEM Incubation Cleanup
The emulsion is broken by addition of a recovery agent and the amplified fragments are purified.
4. ATAC Library preparation
Illumina indexes are added during PCR in bulk.
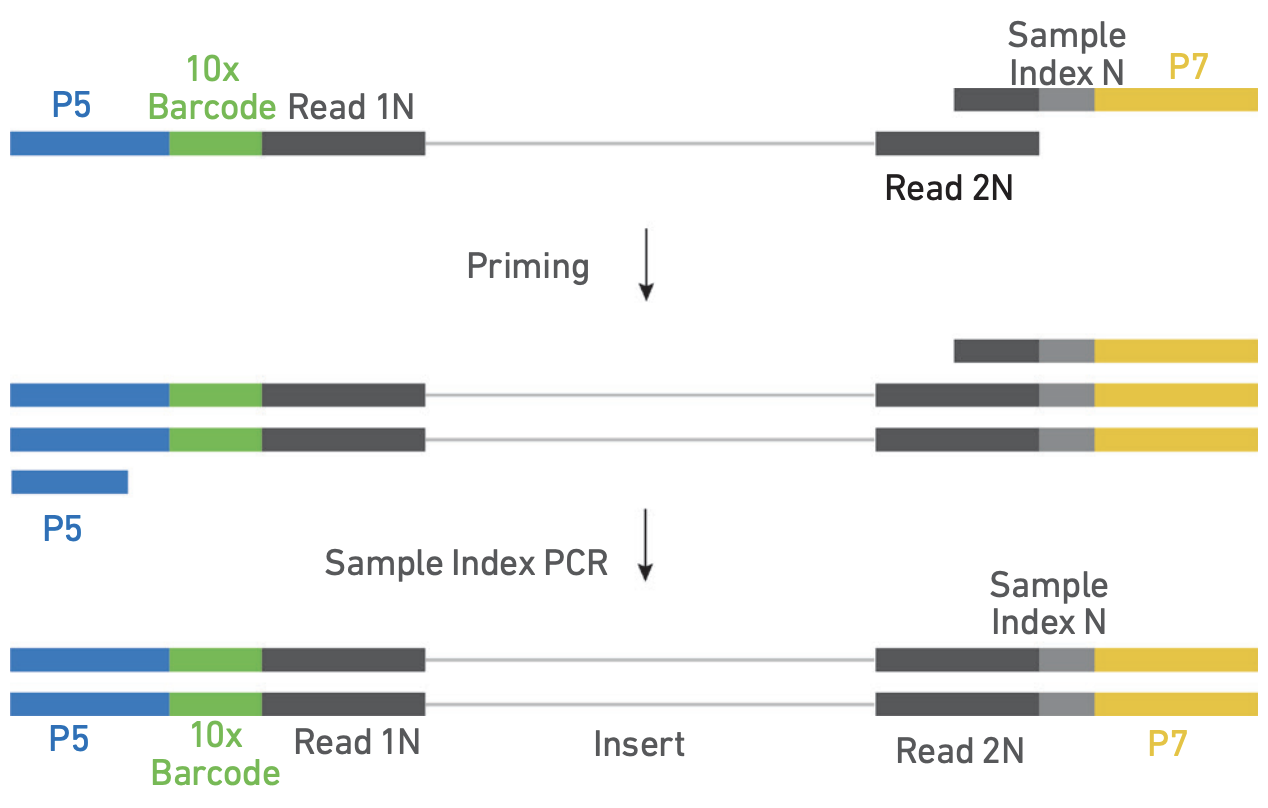
Library construction. Adapted from https://cdn.10xgenomics.com/image/upload/v1666737555/support-documents/CG000496_Chromium_NextGEM_SingleCell_ATAC_ReagentKits_v2_UserGuide_RevB.pdf
5. Library QC and Sequencing
The ATAC library is QCed by Qubit, bioanalyzer and qPCR before pooling for sequencing.

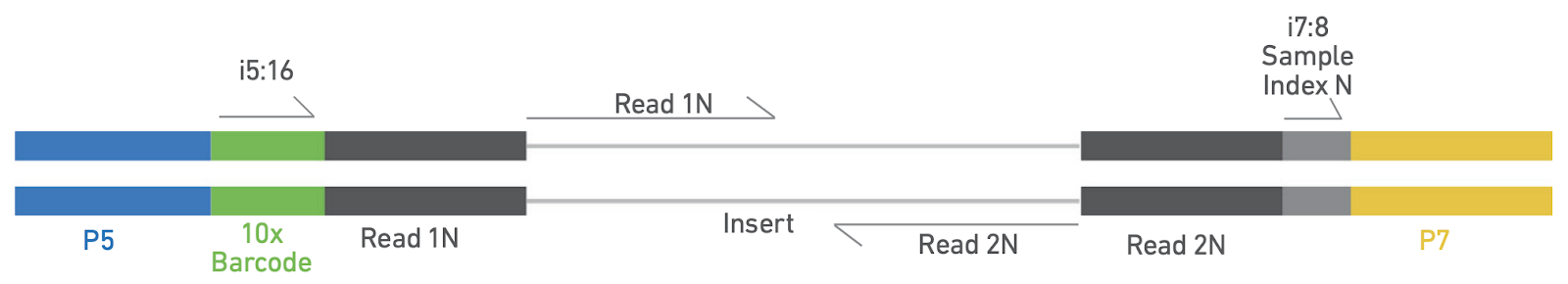
Representative trace of a scATAC library (top). Library structure (bottom). Adapted from https://cdn.10xgenomics.com/image/upload/v1666737555/support-documents/CG000496_Chromium_NextGEM_SingleCell_ATAC_ReagentKits_v2_UserGuide_RevB.pdf
Bioinformatics
Basic bioinformatic analysis using the CellRanger software from 10X Genomics. Your data will be delivered to you through the DDS online delivery system.
Last Updated: 20th May 2024